Search

Find out what our communities had to say about the draft Statement on Consumers and Community Involvement in Health and Medical Research.

The Wesfarmers Centre of Vaccines & Infectious Diseases brings together a number of independent researchers and research teams with a common aim; to find and deliver new and improved solutions to prevent and treat serious infections experienced by children or adolescents.
At the Wesfarmers Centre, we undertake research in five key areas of infections and immunisation to assist in children's health.
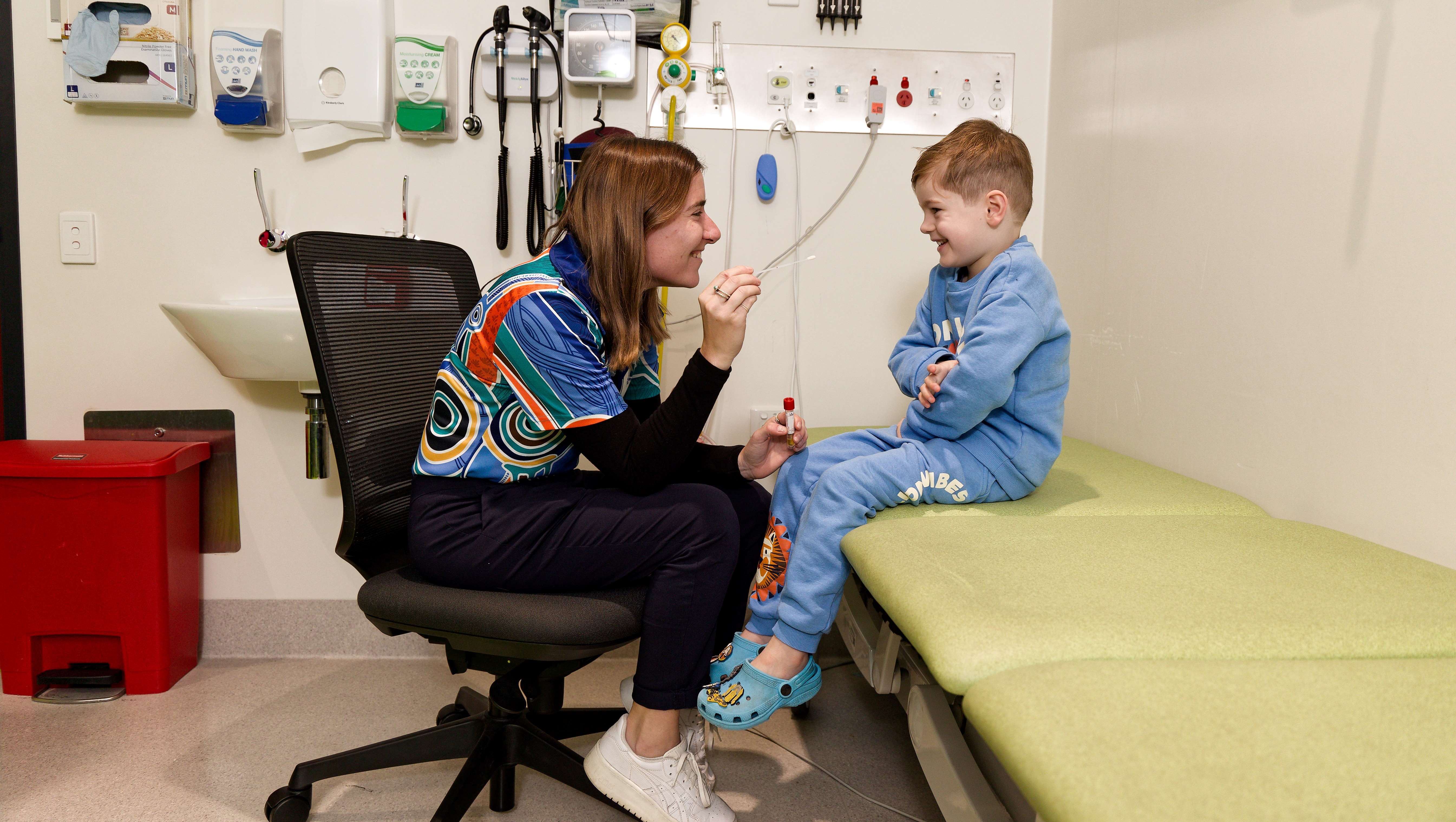
Researchers from The Kids Research Institute Australia would like to understand more about respiratory syncytial virus (RSV) and how we can provide the best protection for kids.
Review the hospital-based research that the Wesfamers Centre of Vaccines & Infectious Diseases conducts.
We are evaluating new vaccines for a range of diseases including influenza, pneumococcal, meningococcal and common infections such as otitis media (glue ear).
Vaccine Trials Group with Sir Charles Gairdner Hospital is conducting a trial of a vaccine against Clostridium difficile infection (CDI) in at-risk individuals.
The study aims to determine whether an RSV vaccine given to pregnant women during the third trimester can protect newborn babies from RSV infections.

Through co-design with community members, we hope to better understand the strengths and effectiveness of community-driven health promotion resources.
The aim of the study is the early identification of problems with the current flu vaccines, and providing parents and professionals with up to date information.
